55502603
Brennstoffzelle
In 10 interaktiven Aufgaben und interaktiven Videos wird Wissen zu Brennstoffzellen vermittelt und anschließend abgefragt.
Das Medium bietet H5P-Aufgaben an, die ohne zusätzliche Software verwendbar sind.
Durch interaktive Aufgabentypen wird das audiovisuelle und interaktive Lernen einfach.
Lernen macht jetzt Spaß!
Included Tasks
- I Energiequellen - Video mit interaktiven Aufgaben
- II Was ist eine Brennstoffzelle? - Lückentext
- III Wirkprinzip: kalte Verbrennung - interaktives Video
- IV Vorteile der Brennstoffzelle - interaktive Aufgabe
- V Elektrolyse und Wasserstoffbrennzelle - interaktive Aufgaben
- VI Kraft-Wärme-Kopplung und Brennstoffzelle - interaktiver Vergleich
- VII Geschichte der Brennstoffzelle - interaktive Zeitleiste
- VIII Brennstoffzellen im Alltag - interaktive Aufgabe
- IX Wissenskarten - interaktive Flashcards
- X Brennstoffzellenquiz - interaktive Aufgabe
Curriculum-centred and oriented towards educational standards
Matching
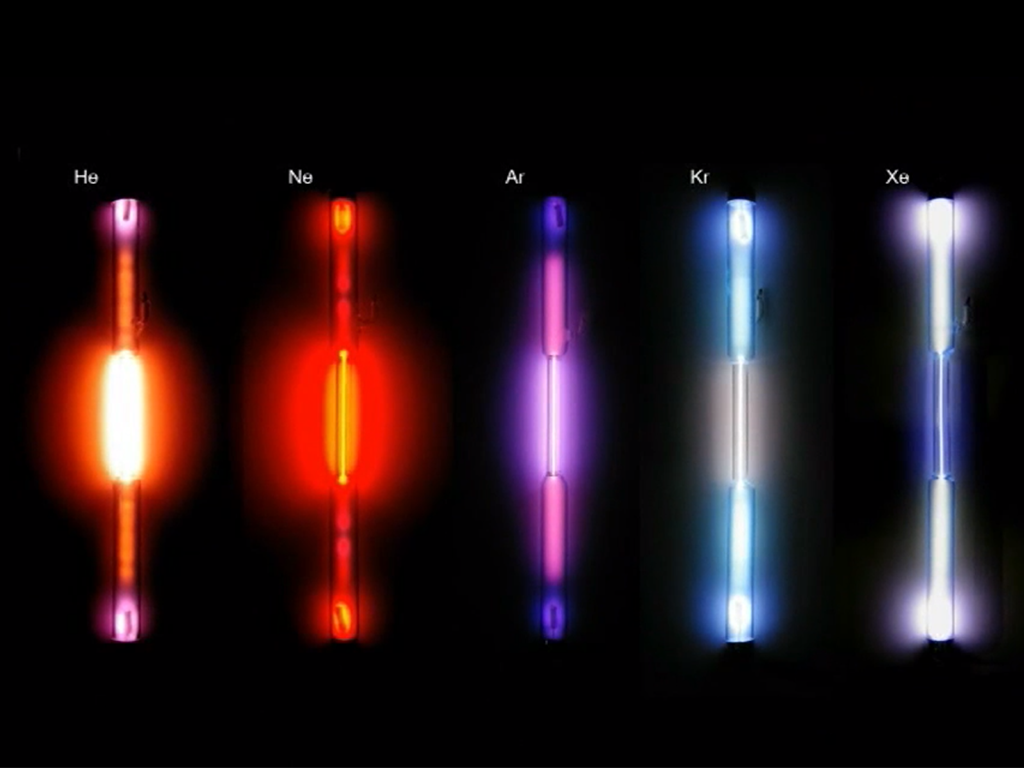
Noble Gases
Xenon, Helium, Neon, Argon, Krypton and the radioactive Radon belong to the noble gases. These form the family of noble gases as the elements of the eighth group of the periodic table. All of them are colourless and odourless, non-inflammable and non-toxic. Their most striking chemical property is their inertness. This can be explained by their electron arrangement, termed noble gas configuration and represents a particularly stable and therefore low-energy state. The noble gases are to be found in scant amounts in our air from which they are also distilled. Helium is mainly extracted from natural gas. In everyday life, we encounter noble gases for example as shielding, filling or buoyant gases and in fluorescent tubes. The shell model describes the structure of the atoms. It is based on the distribution of electrons in restricted areas at a fixed distance around the core of the atom.
Aluminium I
In the modern world, we encounter aluminium at every turn. This is due to the particular properties of the metal. Increasingly, aluminium is about to edge iron and steel out of engineering, as aluminium allows energy-saving lightweight construction of aircraft and vehicles of all kind. Aluminium is weather-resistant, does not rust and is therefore well suited as building material for house facades, window frames or simply for all parts that are exposed to wind and weather. At the same time, aluminium has a noble-looking surface recommending it as material for interior design.
Basics of Chemistry II
When we take a closer look at substances, we discover that they consist of either one single element or of mixtures of several elements. Chemists therefore divide the world of substances into pure and mixed chemical substances. A pure substance is of homogeneous composition. Substance mixtures, however, consist of two or more pure substances. The many mixtures are subdivided not only into homogeneous and heterogeneous mixtures but depending on the respective aggregate states of their components, are classified into various groups of mixtures.