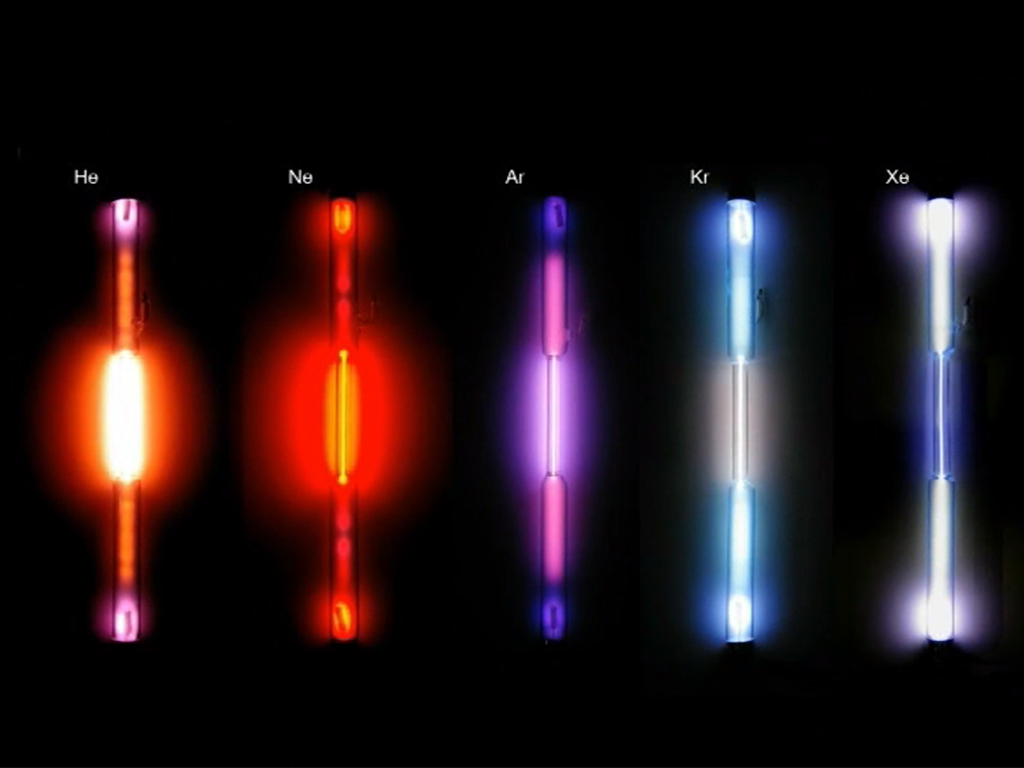
 Noble Gases
Noble Gases
4665872 / 5558097
Noble Gases
A Family of Noble Disposition
Xenon, Helium, Neon, Argon, Krypton and the radioactive Radon belong to the noble gases. These form the family of noble gases as the elements of the eighth group of the periodic table. All of them are colourless and odourless, non-inflammable and non-toxic. Their most striking chemical property is their inertness. This can be explained by their electron arrangement, termed noble gas configuration and represents a particularly stable and therefore low-energy state. The noble gases are to be found in scant amounts in our air from which they are also distilled. Helium is mainly extracted from natural gas. In everyday life, we encounter noble gases for example as shielding, filling or buoyant gases and in fluorescent tubes. The shell model describes the structure of the atoms. It is based on the distribution of electrons in restricted areas at a fixed distance around the core of the atom.
Play trailer
Curriculum-centred and oriented towards educational standards
Matching
Inclusion
Madita is eleven and blind. She does not want to go to a special school but to a regular grammar school. She says she feels "normal" there. Jonathan is eight and has a walking disability. He likes going to the school where he lives. Here, his best friend sits next to him. Max Dimpflmeier, a teacher who is severely deaf, explains that school life is not easy. Quote Max Dimpflmeier: "You don't want to attract attention, you want to avoid saying that it is necessary for you that 70 people adjust to your situation." People on their way to inclusion.
Podcasting
Today, the use of new media has become a matter of course not only in everyday life – schools and teaching, too, benefit from the new technologies and methods, which support active and independent learning. Especially in computer science, ethics and language courses but also in all other subjects, modern media are a valuable pedagogic and didactic asset. This DVD uses the example of podcasts to demonstrate how the possibilities opened up by new media can be applied in the classroom and how the pupils can be taught to handle them in a competent and target-oriented manner. The film is aimed at supporting the use of podcasts at school and encourages making them. This also requires the ability to find information on the Internet and assess it. The film informs on the functionality of podcasts and technical background as well as on the teaching and learning possibilities offered by podcasts – ranging from specific contents to superordinate learning targets such as the advancement of creativity and team spirit. The DVD is a useful support for teachers applying new media and wishing to show their pupils how to handle Running Time: 20:29 ms them in a sensible way.
Youth Movement
Dancing until your feet hurt: Here, at the meeting on the Hoher Meissner near Kassel, 3,500 participants from Boy Scout associations, youth and Wandervogel groups from all over the German-speaking region have gathered. They want to celebrate, simply get to know each other and commemorate a historic anniversary.