Physics
Physics

46503480 / 55502972
States of Aggregation
Solid, Liquid, Gaseous
It is winter. Snow and ice transform the landscape into a fairy tale scenery. At sub-zero temperatures, ice crystals form on branches and other objects.
The snow cover is not always uniform but sometimes shows varied structures of snow and ice crystals as well.
Snow is frozen rain, that is, precipitation falling from the clouds. Clouds, in turn, consist of gaseous water vapour which transforms into precipitation in certain conditions.
Where the sun shines and temperatures rise again, snow and ice begin to melt. Thaw sets in, and snow and ice change into water again. But snow and ice can also exist simultaneously.
Even at high minus temperatures, streams and rivers still carry water. On their banks, exciting ice formations can develop as a result.
Stagnant waters, such as lakes and ponds, however, tend to freeze because the water in them hardly moves.
The solid, liquid and gaseous states are the three classic aggregate states. We encounter the interplay between water vapour, liquid water and ice in many situations.
Complex physical processes lie behind it. Let us take a closer look at them now.

Curriculum-centred and oriented towards educational standards
Matching
Stalking
n Germany, 12 % of all federal citizens are pursued by a stalker once in their lives. And not only celebrities are among their victims! Everyone may be confronted with such a situation.
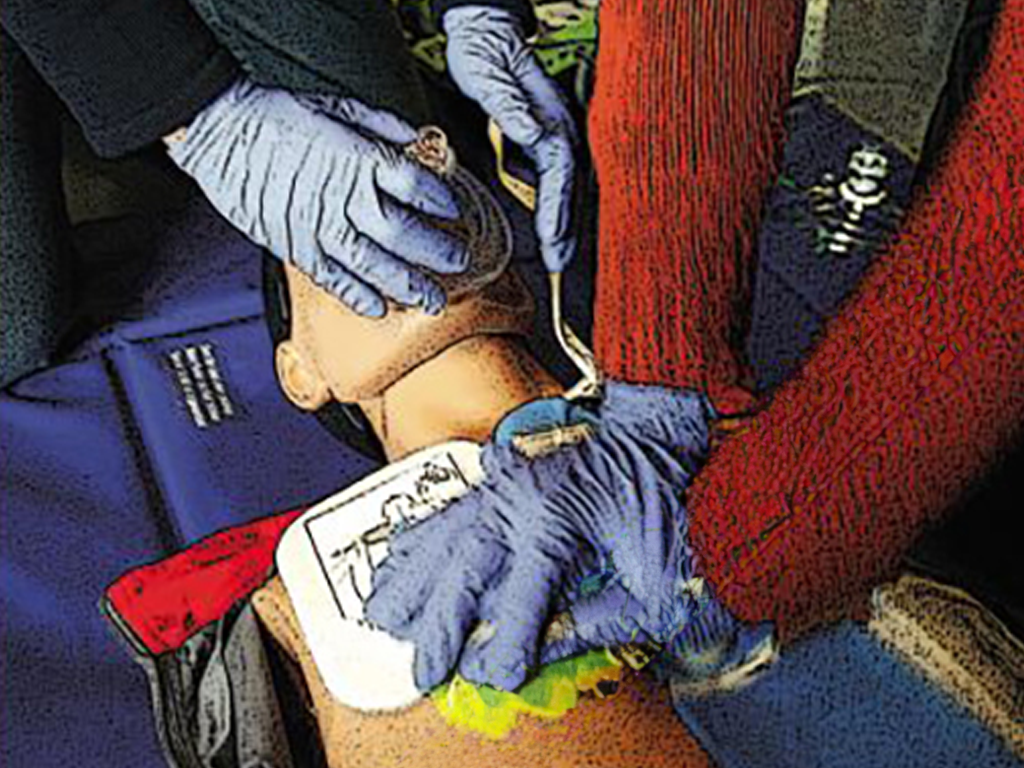
Resuscitation
It can happen to anyone – of any age, in any place, at any time. Sudden cardiac arrest may quickly prove fatal. Immediate action is called for! Just remember: Check Call Press Anyone can do it. You can't do anything wrong!
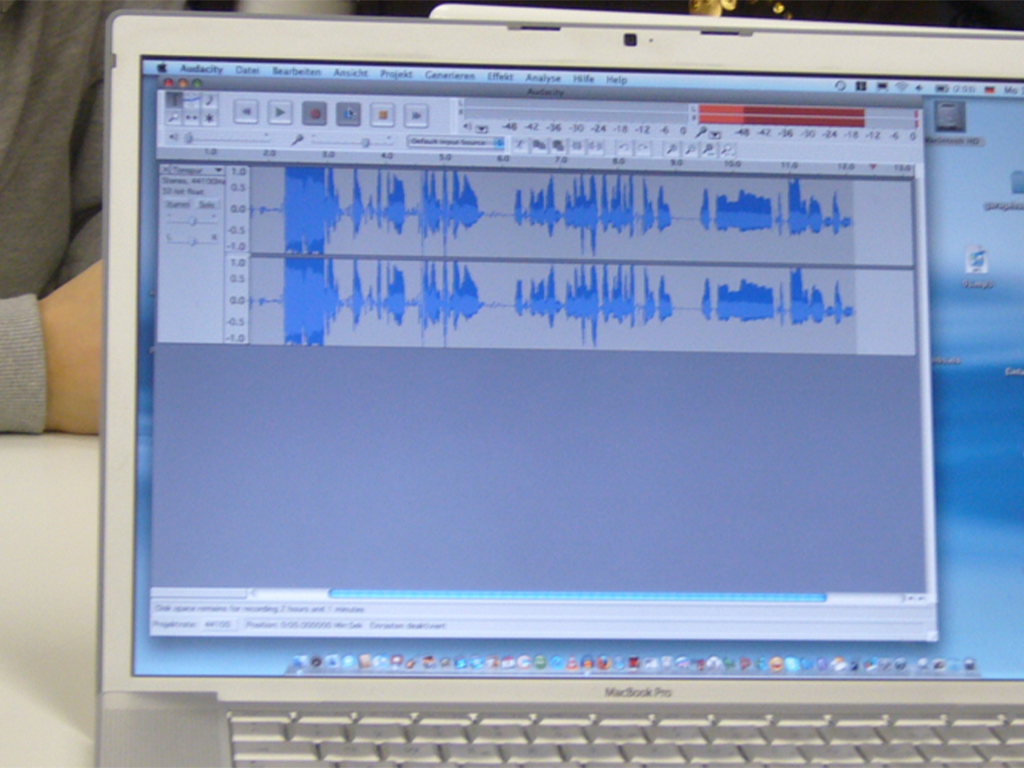
Podcasting
Today, the use of new media has become a matter of course not only in everyday life – schools and teaching, too, benefit from the new technologies and methods, which support active and independent learning. Especially in computer science, ethics and language courses but also in all other subjects, modern media are a valuable pedagogic and didactic asset. This DVD uses the example of podcasts to demonstrate how the possibilities opened up by new media can be applied in the classroom and how the pupils can be taught to handle them in a competent and target-oriented manner. The film is aimed at supporting the use of podcasts at school and encourages making them. This also requires the ability to find information on the Internet and assess it. The film informs on the functionality of podcasts and technical background as well as on the teaching and learning possibilities offered by podcasts – ranging from specific contents to superordinate learning targets such as the advancement of creativity and team spirit. The DVD is a useful support for teachers applying new media and wishing to show their pupils how to handle Running Time: 20:29 ms them in a sensible way.