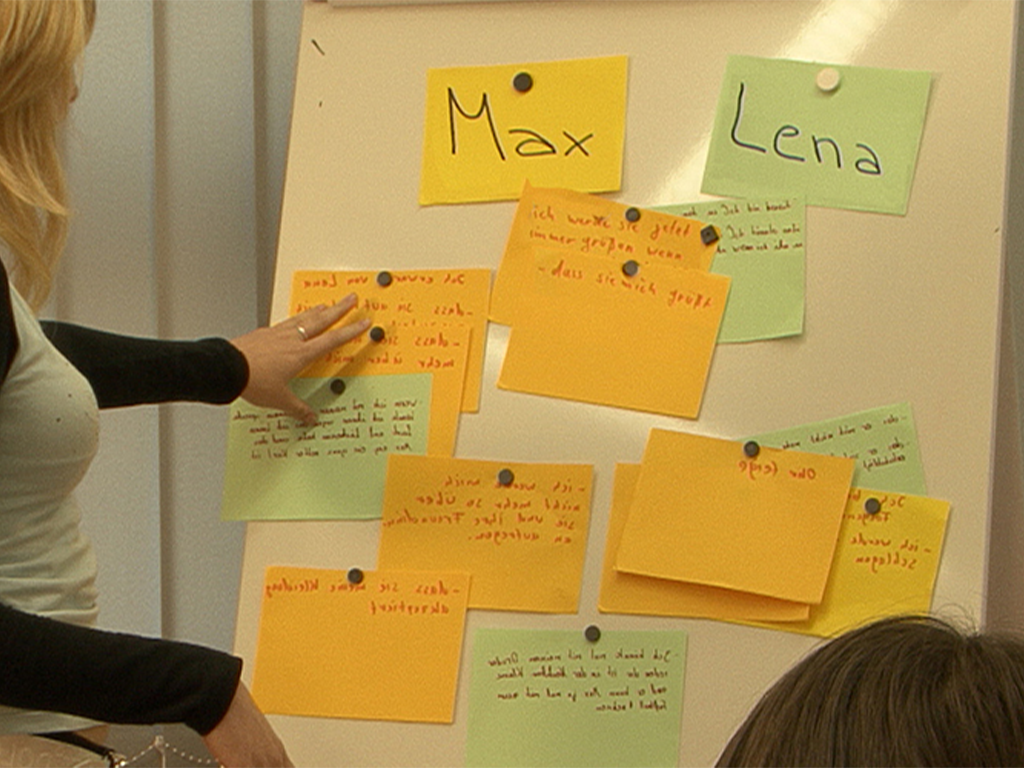Chemistry
Chemistry
4665872 / 5558097
Noble Gases
A Family of Noble Disposition
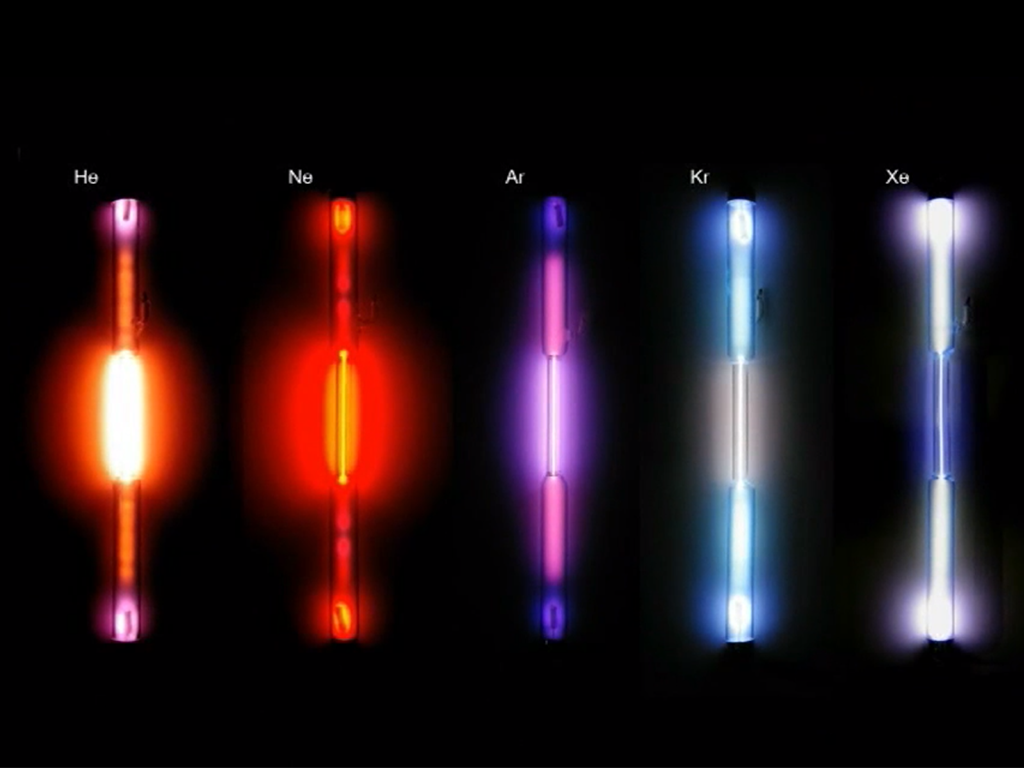
Xenon, Helium, Neon, Argon, Krypton and the radioactive Radon belong to the noble gases. These form the family of noble gases as the elements of the eighth group of the periodic table. All of them are colourless and odourless, non-inflammable and non-toxic. Their most striking chemical property is their inertness. This can be explained by their electron arrangement, termed noble gas configuration and represents a particularly stable and therefore low-energy state. The noble gases are to be found in scant amounts in our air from which they are also distilled. Helium is mainly extracted from natural gas. In everyday life, we encounter noble gases for example as shielding, filling or buoyant gases and in fluorescent tubes. The shell model describes the structure of the atoms. It is based on the distribution of electrons in restricted areas at a fixed distance around the core of the atom.
Play trailer
Curriculum-centred and oriented towards educational standards
Matching
Peer Mediation
Lena and Max attend the 7th form. Max is new in class. During a break, Max notices that Lena and her friend are laughing at him again. Max loses his temper! He slaps Lena in the face. That hurts and Lena runs back into the classroom with a red cheek. The growing conflict between the two has escalated. Just like Lena and Max, every day pupils all over Germany have rows with each other. At the Heinrich Hertz Gymnasium in Thuringia, pupils have been trained as mediators for years. At set hours, they are in a room made available by the school specifically for mediation purposes. The film describes the growing conflict between Max and Lena and shows a mediation using their example. In doing so, the terms “conflict” and “peer mediation” are explained in a non-technical way. The aims of peer mediation and its progress in five steps as well as the mediators’ tasks are illustrated. The art of asking questions and “mirroring”, which the mediators must know, is described and explained. Together with the comprehensive accompanying material, the DVD is a suitable medium to introduce peer mediation at your school, too.
Computer Games
This film covers the topic of computer games in a variety of ways and from many different angles. Apart from the fascina- tion of computer games for users, the historical development as well as the production of computer games are described. The established genres are introduced, the guidelines of the German BPjM are explained. In light of recent public discussions, a neutral overview of the pros and cons of playing computer games is given, and different kinds of player behaviour are outlined. In this film, the pupils will recognise many aspects of their favourite pastime that encourage an independent, constructive use of this medium and reinforce their media competency. The film and teaching material are very closely related to the real-life situation.