55502477
Aluminium
In 10 interaktiven Modulen und in interaktiven Videos wird Wissen zum Thema Aluminium vermittelt und abgefragt.
Das Medium bietet H5P-Aufgaben an, die ohne zusätzliche Software verwendbar sind.
Durch interaktive Aufgabentypen wird das audiovisuelle und interaktive Lernen einfach.
Lernen macht jetzt Spaß!
Included Tasks
- I Steckbrief Aluminium - Interaktive Aufgaben
- II Bauxit - Lückentext
- III Aluminiumherstellung - Bildzuordnung
- IV Vom Bauxit zum Aluminiumoxid - Interaktive Aufgabe
- V Bindungsarten von Aluminium - Interaktives Video
- VI Elektrolyse - Interaktive Aufgabe
- VII Warum rostet Aluminium nicht? - Interaktives Video
- VIII Eloxieren - Bildzuordnung
- IX Vor- und Nachteile von Aluminium - Interaktive Aufgaben
- X Aluminiumrecycling - Interaktive Aufgabe
Curriculum-centred and oriented towards educational standards
Matching
Aluminium I
In the modern world, we encounter aluminium at every turn. This is due to the particular properties of the metal. Increasingly, aluminium is about to edge iron and steel out of engineering, as aluminium allows energy-saving lightweight construction of aircraft and vehicles of all kind. Aluminium is weather-resistant, does not rust and is therefore well suited as building material for house facades, window frames or simply for all parts that are exposed to wind and weather. At the same time, aluminium has a noble-looking surface recommending it as material for interior design.
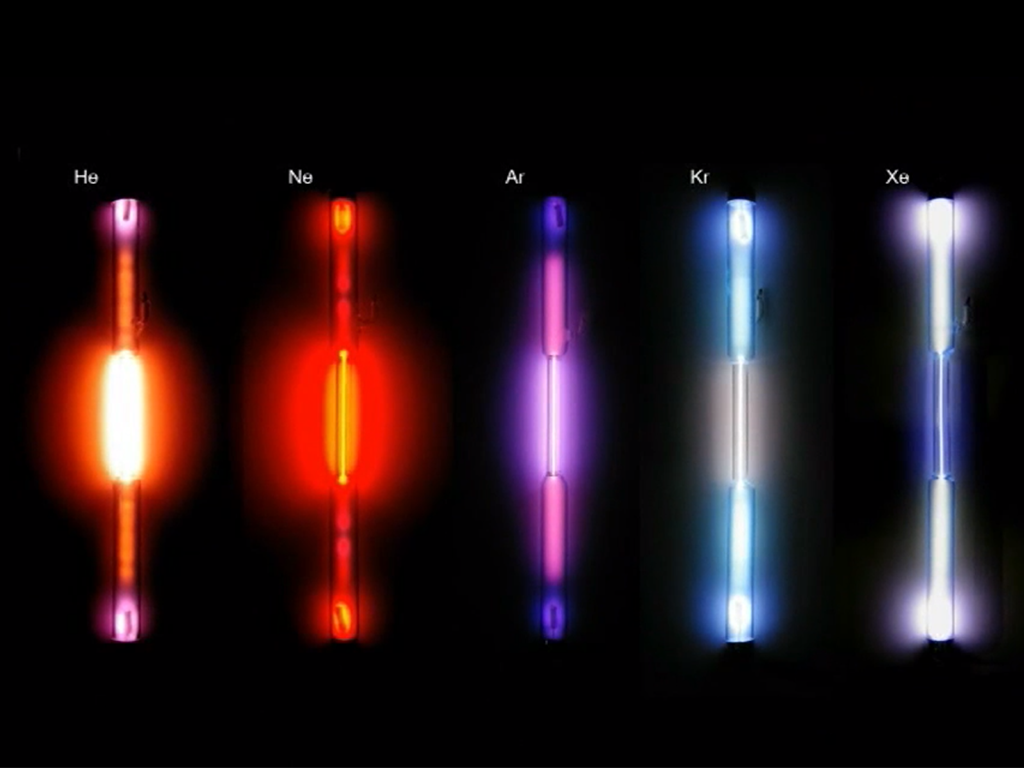
Noble Gases
Xenon, Helium, Neon, Argon, Krypton and the radioactive Radon belong to the noble gases. These form the family of noble gases as the elements of the eighth group of the periodic table. All of them are colourless and odourless, non-inflammable and non-toxic. Their most striking chemical property is their inertness. This can be explained by their electron arrangement, termed noble gas configuration and represents a particularly stable and therefore low-energy state. The noble gases are to be found in scant amounts in our air from which they are also distilled. Helium is mainly extracted from natural gas. In everyday life, we encounter noble gases for example as shielding, filling or buoyant gases and in fluorescent tubes. The shell model describes the structure of the atoms. It is based on the distribution of electrons in restricted areas at a fixed distance around the core of the atom.
C, CO2 and Associates in Everyday Life
All organic matter contains carbon. Coal is deposited in the Earth's interior. It developed about 300 million years ago from plants in a geological period which is also called Carboniferous. During the combustion of organic matter, carbon turns into the gas carbon dioxide. Dissolved in water, it becomes the so-called carbonic acid. Carbon dioxide is an incombustible, colourless and odourless gas that is easily dissolved in water. With various metal oxides or hydroxides it forms two types of salts: the carbonates and the hydrogen carbonates. As calcium carbonate it is contained in natural products such as chalk and egg shells. Specific forms of carbon, called modifications, are graphite and also the particularly valuable diamond.