55502536
Grundlagen chemisches Rechnen 1
In 10 interaktiven H5P-Modulen gibt es Aufgaben zu Berechnungen von Masse, molarer Masse und Stoffmenge.
Das Medium bietet H5P-Aufgaben an, die ohne zusätzliche Software verwendbar sind.
Durch interaktive Aufgabentypen wird das audiovisuelle und interaktive Lernen einfach.
Lernen macht jetzt Spaß!
Included Tasks
- I Formeln; Größen und chemisches Rechnen - Wissensvermittlung
- II Berechnungen mit Unit (u) und Mol (mol) - (1)
- III Berechnungen mit Unit (u) und Mol (mol) - (2)
- IV Berechnungen mit Unit (u) und Mol (mol) - (3)
- V Finde die 7 Elemente - die nie als einzelnes Atom vorliegen
- VI Berechnungen der molaren Masse einer chemischen Verbindung (1)
- VII Berechnungen der molaren Masse einer chemischen Verbindung (2)
- VIII Berechnungen von molarer Masse mit verschiedenen Vorgaben (1)
- IX Berechnungen von molarer Masse mit verschiedenen Vorgaben (2)
- X Gemischte Berechnungen Masse; Stoffmenge; molare Masse
Curriculum-centred and oriented towards educational standards
Matching
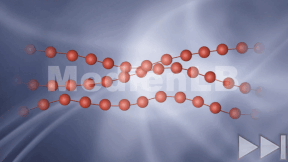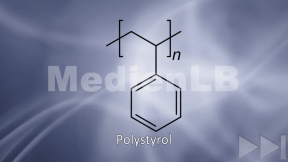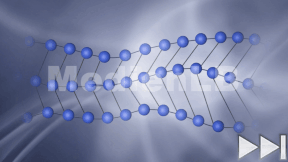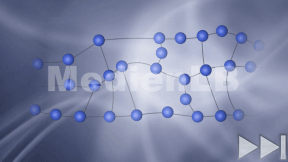
Plastic
Plastic has been around for not longer than roughly 100 years, and the synthetic material is a brilliant invention. Its production is cheap, it can take almost any possible form, it is light-weight, versatile and, above all, inexpensive.
Carbohydrates
The term carbohydrate or saccharide is a collective name for all substances with the chemical formula Cn(H2O)n. Carbohydrates are the basis of nutrition. They are part of our diet as starch, glucose (grape sugar), fructose (fruit sugar), lactose (milk sugar) and saccharose (beet, cane or table sugar). Important suppliers of carbohydrates are potatoes and cereals such as rice, wheat, maize, millet, rye and oats. The various carbohydrates in our foods are introduced to the pupils. The characteristics of polysaccharides, disaccharides and monosaccharides are explained to them and in which foods these substances occur and how they are structured. In addition, the different origins of starch, starch degradation products, gelling agents as well as sugar alcohols in confectionery are dealt with. The DVD shows how various substances can be detected with the help of chemical processes. Together with the extensive accompanying material the DVD is ideally suited for use in the classroom.