55502476
Plastik
In 14 interaktiven Modulen wird das Thema Plastik vermittelt und anschließend abgefragt.
Das Medium bietet H5P-Aufgaben an, die ohne zusätzliche Software verwendbar sind.
Durch interaktive Aufgabentypen wird das audiovisuelle und interaktive Lernen einfach.
Lernen macht jetzt Spaß!
Included Tasks
- I Eigenschaften von Plastik - Interaktives Video
- II Alternativen zum Plastik - Video und interaktive Aufgaben
- III Werkstoff auf Kohlenstoffbasis - Video und interaktive Aufgaben
- IV Arten von Plastik - Bildzuordnung
- V Recycling von Plastik - Interaktives Video
- VI Plastik - Wortsuche
- VII Kunststoff-Recycling - Interaktive Aufgabe
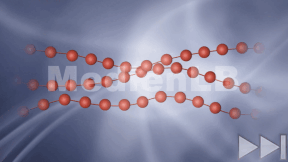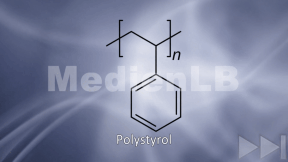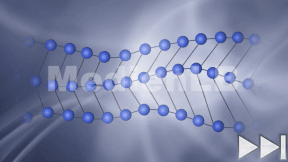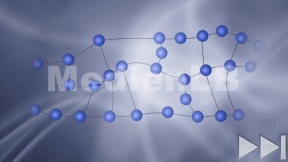
- VIII Struktur der Kunststoffe - Bildzuordnung
- IX Biokunststoff und andere Ideen für die Zukunft - Interaktive Aufgaben
- X Der Grüne Punkt - Interaktive Aufgabe
- XI Vorteile der Verwendung von Kunststoffen - Lückentext
- XII Kunststoffe im Meer - Video mit Aufgaben
- XII Kunststoffverzicht im Alltag - Interaktive Aufgbe
- XIV Kunststoffarten - Interaktive Aufgabe
Curriculum-centred and oriented towards educational standards
Matching
Plastic
Plastic has been around for not longer than roughly 100 years, and the synthetic material is a brilliant invention. Its production is cheap, it can take almost any possible form, it is light-weight, versatile and, above all, inexpensive.
Acids and Bases
We can find acids and bases in every supermarket, some of them in our food, others in cleaning agents. In everyday products, acids and bases as well as acidic and alkaline reacting salts have extremely different functions. In food, acids are either present or added as flavouring agents such as citric acid, tartaric acid and acetic acid, as antioxidants such as ascorbic acid or generally as acidifiers, sequestrants (citric acid and tartaric acid) and preservatives (acetic acid).