55506135
Lerneinheit Chemie 8 – Kalk
In unserem Arbeitsheft „Lerneinheit Chemie 8 – Kalk“ finden Sie 10 interaktive und didaktisch aufbereitete Aufgaben zum Thema Kalk.
Über die Beschreibung der Entstehung und Funktion von Kalk, geben die Aufgaben Hinweis auf seinen Einsatz in der Industrie oder auch im Alltag.
Unterschiedliche Aufgabentypen dienen der Wissensvermittlung, Wissensvertiefung und der Lernstandabfrage.
Die Aufgaben sind mit H5P erstellt und können ohne zusätzliche Software angewandt werden.
Lernen macht jetzt Spaß!
Demo
Included Tasks
- Kalk - Seine Funktionen
- Kalk - Von der Entstehung bis zur Verwitterung
- Kalk - Abbau und Gewinnung
- Kalk - Einsatz in der Industrie
- Kalk im Alltag
- Kalk - Finde die Bildpaare
- Kalk - Schulversuche
- Kalk - Suchsel
- Kalk - Kreuzworträtsel
- Kalk - Teste dein Wissen
Curriculum-centred and oriented towards educational standards
Matching
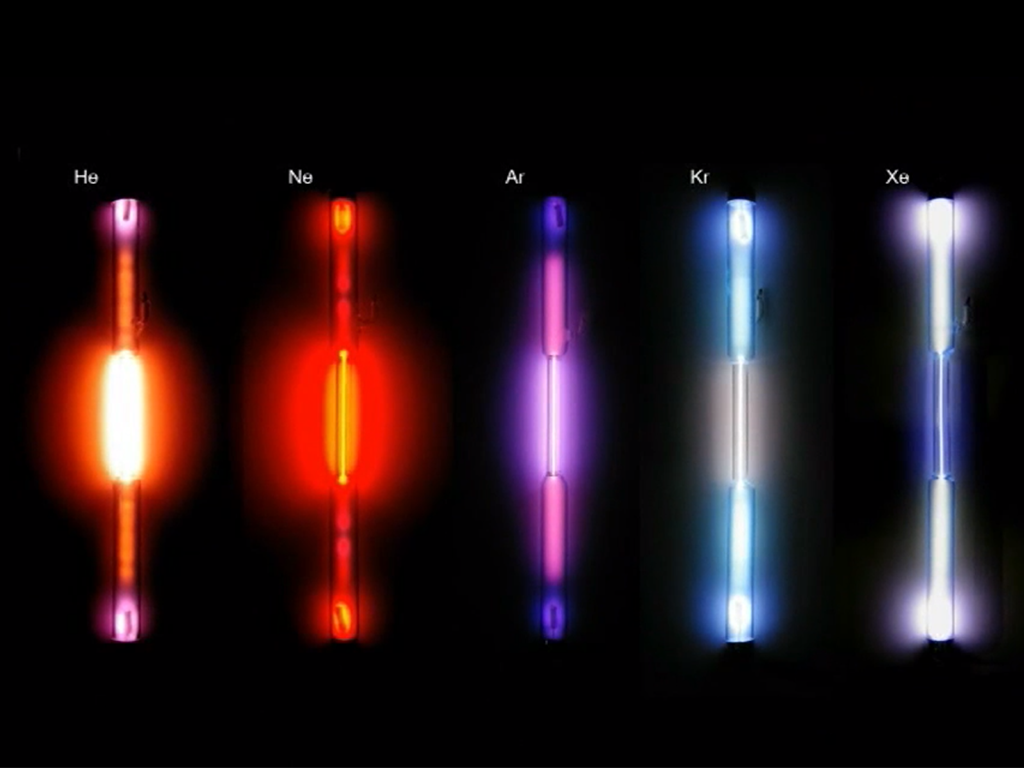
Noble Gases
Xenon, Helium, Neon, Argon, Krypton and the radioactive Radon belong to the noble gases. These form the family of noble gases as the elements of the eighth group of the periodic table. All of them are colourless and odourless, non-inflammable and non-toxic. Their most striking chemical property is their inertness. This can be explained by their electron arrangement, termed noble gas configuration and represents a particularly stable and therefore low-energy state. The noble gases are to be found in scant amounts in our air from which they are also distilled. Helium is mainly extracted from natural gas. In everyday life, we encounter noble gases for example as shielding, filling or buoyant gases and in fluorescent tubes. The shell model describes the structure of the atoms. It is based on the distribution of electrons in restricted areas at a fixed distance around the core of the atom.
Aluminium II
The metal aluminium is growing in importance because of its specific properties and manifold application possibilities. This DVD deals with the industrial production of aluminium as a raw material, its processing and the manufacturing of alloys for the finished product. Starting with the raw material aluminium oxide the functioning of an electrolytic cell is demonstrated and explained. Alumina, white and powdery, is melted with great expenditure of energy, and by means of electrolysis converted into aluminium with a degree of purity of 99.9%. As aluminium oxide would not melt before a temperature of over 2,000°C is reached, the mineral cryolite is used as a solvent. The various alloys change the properties of aluminium and are produced according to precise formulations. The alloy is cast into blocks and bars that serve as primary material for processing plants. The responsible handling of resources underscores the importance of recycling. Aluminium is resilient and versatile.