Chemistry
Chemistry

55502480
Eigenschaften von Stoffen
In 10 interaktiven Modulen und in interaktiven Videos wird Wissen zu den Themen Stoffe und ihre Eigenschaften, Gemische, Gemenge und Lösungen vermittelt und abgefragt.
Das Medium bietet H5P-Aufgaben an, die ohne zusätzliche Software verwendbar sind.
Durch interaktive Aufgabentypen wird das audiovisuelle und interaktive Lernen einfach.
Lernen macht jetzt Spaß!
Included Tasks
- I Chemische Eigenschaften - Interaktive Aufgabe
- II Teilchenmodell - Interaktive Aufgaben
- III Dichte - Lückentext
- IV Verständnis durch Modelle - Interaktives Video
- V Härteskala nach Mohs - Interaktive Aufgabe
- VI Überall Gemische - Interaktives Video
- VII Gemenge - Wortgitter
- VIII Reinstoffe und Gemische - Bildzuordnung
- IX Destillation - Interaktive Aufgaben
- X Löslichkeit - Lückentext
Curriculum-centred and oriented towards educational standards
Matching
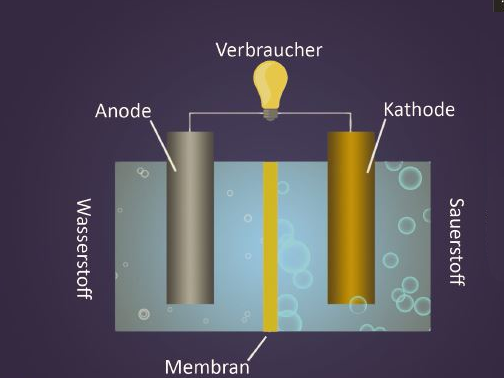
Fuel Cell
A smartphone offers a lot of opportunities nowadays. The numerous apps and applications may enrich your daily life but cost a lot of electricity. It is particularly annoying when the device fails at the most inconvenient moments. Conventional rechargeable batteries are often empty after one day already, and the device needs to be plugged in. Besides many others, also this problem could be solved by using fuel cells – thus considerably increasing the duration of the smartphone.
Halogens
The compounds of halogens are - with the exception of astatine - widespread, can be encountered in nature and are versatile substances. This fact is taken up on this DVD in order to teach the students the chemistry of the halogens by illustrating their special qualities and explaining the correlation of their structure with their chemical properties. In the first part, an overview of the element group of halogens lays emphasis on the common as well as on the distinguishing characteristics of fluorine, chlorine, bromine and iodine. In a second part, the specific properties of fluorine and chlorine are presented. This topic is linked to the students‘ everyday experience (fluorine as a protection against caries, chlorine as a disinfectant, etc.) on the one hand. On the other hand, the DVD presents carefully selected experiments. As a rule, they are of a kind that can only be realized with difficulty, or high expenditure in the chemistry classroom. With the help of these experiments, students are introduced to the chemistry of the halogens in a way that enables them to draw conclusions on the basis of their observations.